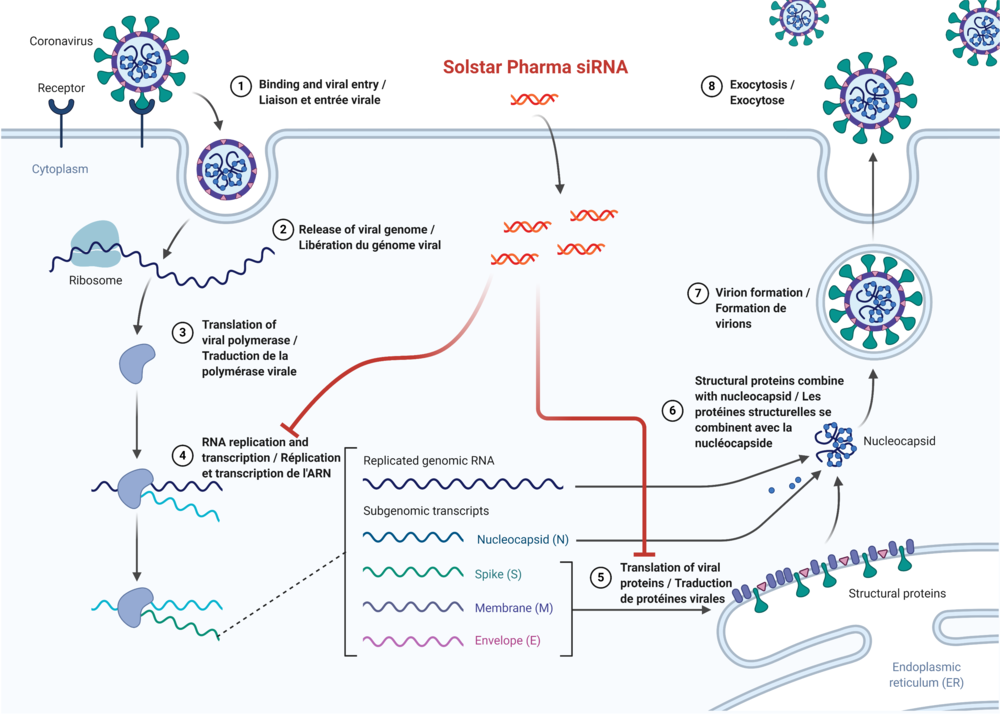
Solstar Pharma, Inc. has filed two International Patent Cooperation Treaty (PCT) applications for coverage of the invention titled, “Antiviral Silencing RNA molecules, Chemically Modified Antiviral Silencing RNA Molecules With Enhanced Cell Penetrating Abilities, Pharmaceutical Compositions Comprising Same And Uses Thereof For Treatment Of Viral Infections” with a filing date of September 3rd, 2021 and “Water-Soluble Artesunate-Based Therapy For Coronavirus Infection” with a filing date of June 23, 2021. The Patent Cooperation Treaty has over 150 participating countries and allows an inventor to seek patent protection in numerous countries simultaneously. Both PCT applications are focused on COVID-19 treatments under development.
In collaboration with GF Mille (GFM) of Japan, the siRNA technology developed to inhibit SARS-Cov-2 viral replication has the potential to treat other viral infections and even aggressive cancers. GFM’s proprietary modified nucleoside technology is at the foundation of these novel modified RNA compounds.
“As we continue to broaden our capabilities in research & development, the collaboration with GF Mille gives us access to breakthrough innovation potential,” said Dr. Dennis Baltzis, President of Solstar Pharma. “These novel RNA drugs will be important to develop new treatments for various types of cancer and infectious diseases, including COVID-19 infections, that will be used for patients with limited treatment options or that fail standard therapy.”
Furthermore, Solstar Pharma’s water-soluble artesunate (WS-ART) has the potential to treat COVID-19. WS-ART has shown to have antiviral effects against SARS-CoV-2 in vitro and artesunate has previously been shown to have anti-inflammatory properties. Recently, the WHO announced the use of intravenous artesunate in its Solidarity clinical trial. Solstar Pharma’s WS-ART is being developed as an oral formulation to be administered as a tablet.
“We are in the process of finalizing our pharmacokinetic and in vivo COVID-19 studies with WS-ART. Together, these data, in conjunction with the WHO clinical trial, we may be able to develop and commercialize our pill form formulated-artesunate,” said Dr. Baltzis. “This will be extremely helpful for developing countries in need of treatments or who can’t afford COVID-19 vaccines”.
Both siRNA and WS-ART compounds are currently being developed and investigated for efficacy and toxicity in vivo. Solstar Pharma and its collaborators are planning to make future announcements regarding these studies in the near future.